Embark on a captivating journey into the realm of radioactive dating game lab answers, where scientific precision meets historical intrigue. This comprehensive guide unravels the mysteries of time, providing a profound understanding of the techniques, applications, and advancements that have revolutionized our comprehension of Earth’s history and human evolution.
From the groundbreaking discoveries of Marie Curie to the latest innovations in isotopic analysis, radioactive dating has transformed our understanding of the past, present, and future. Join us as we delve into the fascinating world of radioactive dating, empowering you with the knowledge to decipher the secrets of time.
1. Historical Context of Radioactive Dating: Radioactive Dating Game Lab Answers
Radioactive dating emerged as a groundbreaking technique in the early 20th century, revolutionizing our understanding of Earth’s history and human evolution. The concept originated with the discovery of radioactivity by Henri Becquerel in 1896, followed by the pioneering work of Ernest Rutherford and Frederick Soddy on radioactive decay and the identification of isotopes.
In 1907, Bertram Boltwood applied radioactive dating to uranium-lead minerals, marking the first successful application of this technique. Subsequently, Willard Libby developed the carbon-14 dating method in the 1940s, which proved invaluable in dating archaeological and organic materials. These advancements laid the foundation for the widespread use of radioactive dating in various fields.
Contributions of Key Scientists, Radioactive dating game lab answers
- Henri Becquerel:Discovered radioactivity in 1896.
- Ernest Rutherford:Coined the term “half-life” and developed the concept of radioactive decay.
- Frederick Soddy:Proposed the concept of isotopes and developed the displacement law of radioactive elements.
- Bertram Boltwood:First applied radioactive dating to uranium-lead minerals in 1907.
- Willard Libby:Developed the carbon-14 dating method in the 1940s.
Initial Applications and Limitations
Early applications of radioactive dating focused on geological dating, including the determination of the age of rocks and minerals. It also proved useful in archaeology, providing insights into the chronology of ancient artifacts and human settlements.
However, limitations existed in the accuracy and precision of early dating methods. The development of more sophisticated techniques and the use of multiple isotopes have since improved the reliability of radioactive dating.
2. Principles of Radioactive Dating
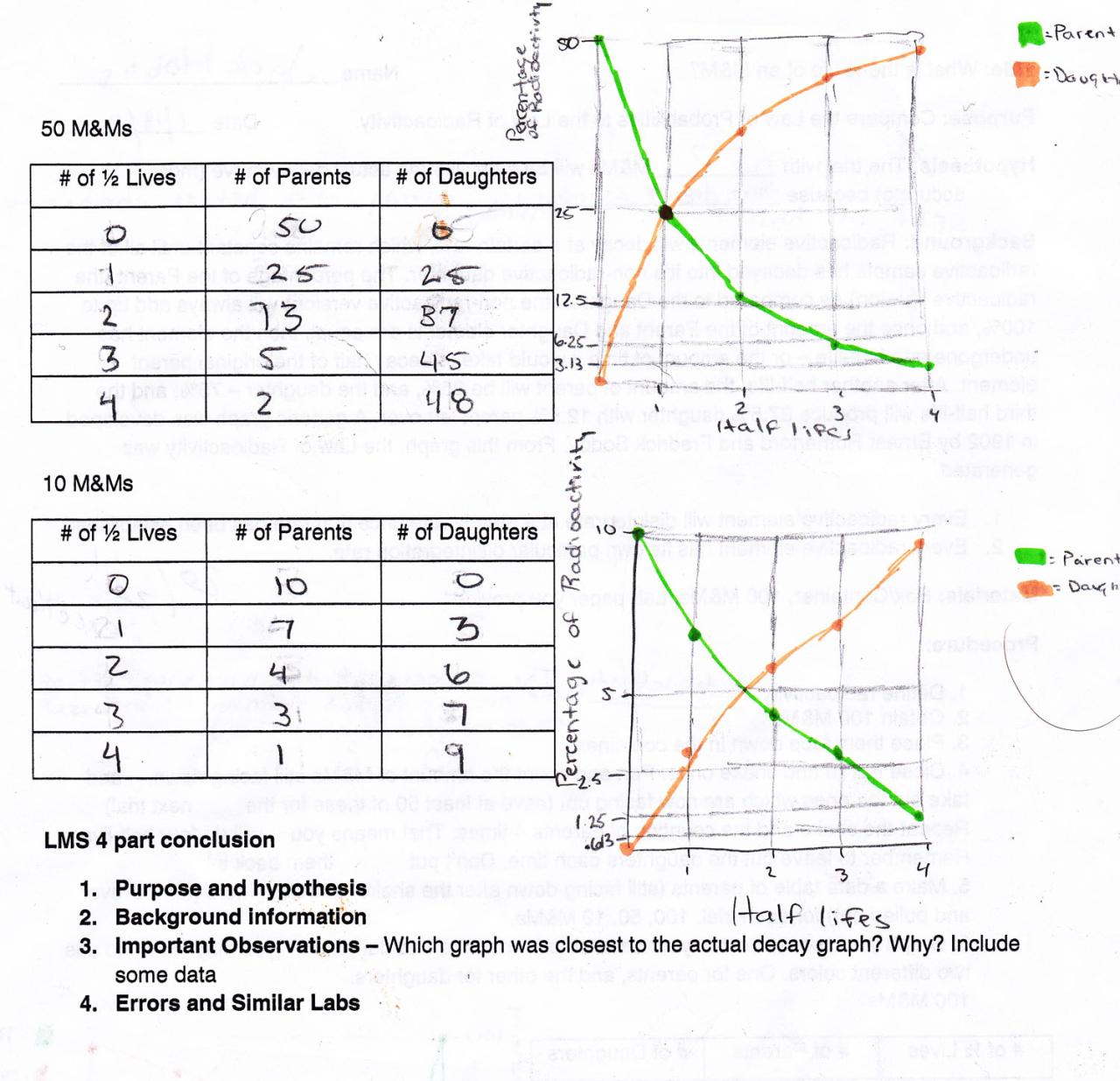
Radioactive dating relies on the concept of radioactive decay, a process in which unstable isotopes undergo spontaneous transformation, emitting particles and energy. The rate of decay is constant for each isotope, known as its half-life, which represents the time it takes for half of the radioactive atoms in a sample to decay.
Different types of radioactive isotopes are used in dating, each with specific characteristics. Carbon-14, with a half-life of 5,730 years, is ideal for dating organic materials up to 50,000 years old. Potassium-40, with a half-life of 1.25 billion years, is suitable for dating rocks and minerals.
Uranium-238, with a half-life of 4.47 billion years, is used for dating geological formations.
Decay Rates and Age Determination
The decay rate of a radioactive isotope is inversely proportional to its half-life. By measuring the ratio of the parent isotope to the daughter isotope in a sample, scientists can determine the age of the material.
For example, in carbon-14 dating, the ratio of carbon-14 to carbon-12 in a sample is measured. As carbon-14 decays over time, the ratio decreases. By comparing the ratio to known standards, the age of the sample can be calculated.
FAQ Explained
What is the principle behind radioactive dating?
Radioactive dating relies on the concept of radioactive decay, where unstable isotopes undergo spontaneous transformations at a constant rate. By measuring the ratio of parent to daughter isotopes in a sample, scientists can determine its age.
What are the different types of radioactive isotopes used in dating?
Commonly used isotopes include carbon-14 for dating organic materials, potassium-40 for dating geological formations, and uranium-238 for dating rocks and minerals.
How accurate is radioactive dating?
The accuracy of radioactive dating depends on the technique used and the age of the sample. Carbon-14 dating, for instance, is highly accurate for samples up to 50,000 years old, while uranium-lead dating can date samples billions of years old.