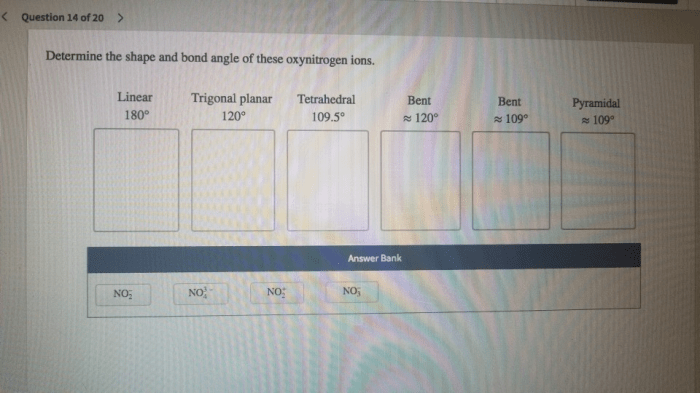
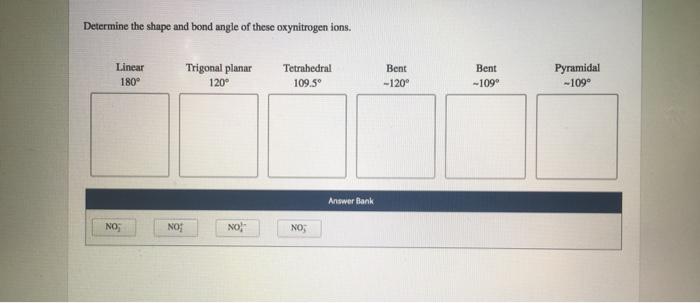
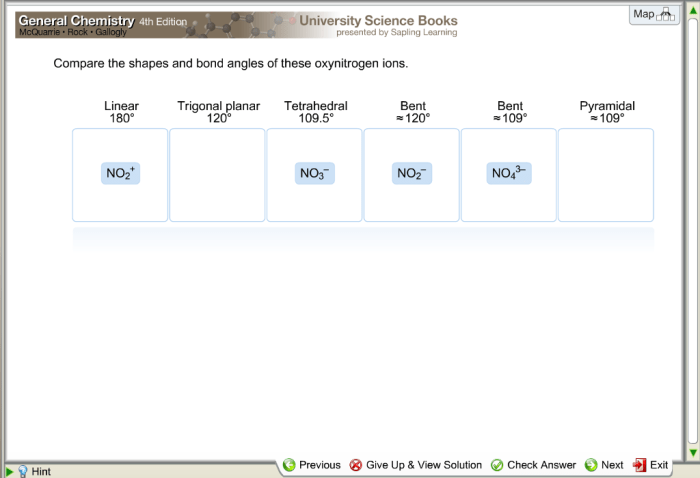
Determine the shape and bond angle of these oxynitrogen ions – Determining the shape and bond angle of oxynitrogen ions is crucial in understanding their chemical and physical properties. This comprehensive analysis delves into the intricacies of molecular geometry, VSEPR theory, and experimental techniques to unravel the structural characteristics of these fascinating ions.
Oxynitrogen ions exhibit diverse geometries, ranging from linear to tetrahedral, influenced by factors such as the number and arrangement of electron pairs. Understanding these geometries is essential for predicting their reactivity, polarity, and solubility, ultimately shaping their role in various chemical processes.
Structural Analysis of Oxynitrogen Ions
The geometry of oxynitrogen ions plays a crucial role in determining their chemical and physical properties. Understanding the molecular geometry and bond angles of these ions is essential for predicting their reactivity, polarity, and other important characteristics.
Molecular Geometry and VSEPR Theory
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting the geometry of molecules based on the number of valence electron pairs around the central atom.
Common Oxynitrogen Ion Geometries, Determine the shape and bond angle of these oxynitrogen ions
- Linear:NO 2–, NO 3–(2 valence electron pairs)
- Bent:NO 2(3 valence electron pairs)
- Trigonal Planar:NO 32-(4 valence electron pairs)
- Tetrahedral:NO 43-(5 valence electron pairs)
The geometry of oxynitrogen ions is influenced by the number of valence electrons and the electronegativity of the atoms involved.
Bond Angle Determination
Bond angles can be determined using various experimental and computational techniques, including:
- X-ray crystallography:Measures the positions of atoms within a crystal, providing information about bond lengths and angles.
- Electron diffraction:Determines the scattering pattern of electrons passing through a sample, allowing for the calculation of bond angles.
- Computational methods:Uses quantum mechanics to predict the geometry and bond angles of molecules based on their electronic structure.
Impact of Geometry on Properties
The geometry of oxynitrogen ions has a significant impact on their properties:
- Polarity:The shape of an ion affects its charge distribution, leading to differences in polarity and reactivity.
- Solubility:The geometry of an ion influences its ability to form hydrogen bonds with water molecules, affecting its solubility.
- Reactivity:The bond angles and distances within an ion can affect the accessibility of the active sites, influencing its reactivity.
Q&A: Determine The Shape And Bond Angle Of These Oxynitrogen Ions
What is the significance of molecular geometry in oxynitrogen ions?
Molecular geometry determines the spatial arrangement of atoms in an ion, influencing its bond angles, polarity, and reactivity.
How does VSEPR theory aid in predicting the geometry of oxynitrogen ions?
VSEPR theory considers the electron-pair repulsion to predict the most stable molecular geometry that minimizes electrostatic repulsion.
What experimental techniques are used to determine bond angles in oxynitrogen ions?
Techniques such as X-ray crystallography, microwave spectroscopy, and infrared spectroscopy provide experimental data for bond angle determination.