Chapter 25 nuclear chemistry answer key – Embark on a journey into the fascinating realm of nuclear chemistry with our comprehensive answer key for Chapter 25. This guide provides a roadmap through the intricate world of nuclear reactions, radioactive decay, and the practical applications that shape our understanding of the atom.
Delve into the fundamental principles of nuclear reactions, exploring their types, energy release, and practical applications. Discover the intricacies of radioactive decay, unraveling the mysteries of half-life, decay modes, and their significance in various fields.
Nuclear Reactions: Chapter 25 Nuclear Chemistry Answer Key
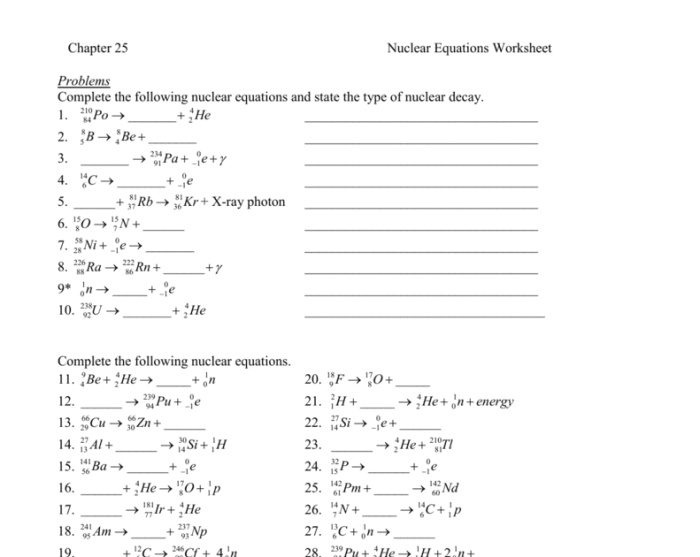
Nuclear reactions involve changes in the structure of atomic nuclei, releasing large amounts of energy. These reactions can be classified into several types based on the nature of the interactions and the particles involved.
Types of Nuclear Reactions
- Nuclear Fission:The splitting of a heavy nucleus into two or more lighter nuclei, releasing energy.
- Nuclear Fusion:The combination of two or more light nuclei into a heavier nucleus, releasing energy.
- Nuclear Transmutation:The conversion of one element into another by changing the composition of the nucleus.
- Nuclear Capture:The absorption of a neutron or other particle by a nucleus, resulting in the formation of a new nucleus.
Energy Released in Nuclear Reactions
Nuclear reactions release significant amounts of energy due to the conversion of mass into energy, as described by Einstein’s famous equation E=mc². This energy is harnessed in nuclear power plants for electricity generation and in nuclear weapons for destructive purposes.
Radioactive Decay
Radioactive decay refers to the spontaneous disintegration of unstable atomic nuclei, emitting radiation and transforming into a more stable form. The rate of decay is characterized by the half-life of the isotope.
Types of Radioactive Decay
- Alpha Decay:Emission of an alpha particle (two protons and two neutrons) from the nucleus.
- Beta Decay:Emission of a beta particle (an electron or positron) from the nucleus, accompanied by a change in atomic number.
- Gamma Decay:Emission of a gamma ray (high-energy photon) from the nucleus, without changing the atomic number or mass.
Half-Life of a Radioactive Isotope
The half-life of a radioactive isotope is the time it takes for half of the atoms in a sample to decay. It is a constant value for each isotope and is used to determine the age of materials and the effectiveness of radioactive tracers.
Applications of Nuclear Chemistry
Nuclear chemistry has a wide range of applications in various fields, including medicine, industry, and environmental science.
Medical Applications
- Radioactive Tracers:Used to diagnose and monitor various medical conditions by tracking the distribution of radioactive isotopes in the body.
- Radiation Therapy:High-energy radiation is used to destroy cancerous cells and shrink tumors.
Industrial Applications
- Nuclear Power:Nuclear fission reactions are used to generate electricity in nuclear power plants.
- Radioactive Gauges:Used to measure the thickness, density, or level of materials in industrial processes.
Environmental Applications, Chapter 25 nuclear chemistry answer key
- Radioactive Waste Disposal:Nuclear waste must be safely disposed of to prevent environmental contamination.
- Nuclear Forensics:Used to investigate the origin and type of radioactive materials in environmental samples.
Nuclear Waste Management

Nuclear waste, a byproduct of nuclear power generation and medical applications, requires proper management to ensure the safety of the environment and the public.
Methods of Nuclear Waste Disposal
- Deep Geological Disposal:Burial of nuclear waste in deep underground geological formations.
- Reprocessing:Separating and reusing usable materials from nuclear waste, reducing its volume and radioactivity.
Challenges of Nuclear Waste Management
- Long-Term Safety:Ensuring the safety of nuclear waste for thousands of years.
- Public Perception:Overcoming public concerns and gaining acceptance for nuclear waste disposal facilities.
Helpful Answers
What are the different types of nuclear reactions?
Nuclear reactions can be classified into various types, including fission, fusion, alpha decay, beta decay, and gamma decay.
What is the half-life of a radioactive isotope?
The half-life of a radioactive isotope represents the time it takes for half of the radioactive atoms in a sample to decay.
What are the medical applications of nuclear chemistry?
Nuclear chemistry plays a vital role in medical imaging, cancer treatment, and the sterilization of medical devices.